Researchers at Wake Forest University of School of Medicine have received a five-year, $1.5 million grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health (NIH), to study bone microarchitecture in patients following bariatric surgery.
With the funding support, researchers at Wake Forest University School of Medicine will partner with Virginia Tech to add a virtual biopsy that uses an innovative technique called high-resolution peripheral quantitative computed tomography (HR-pQCT) to an ongoing randomized controlled trial.
Strategies to Reduce the Onset of Sleeve Gastrectomy Associated Bone Loss (STRONG BONES), is a clinical trial that is testing whether risedronate, an osteoporosis medicine, can minimize bone and muscle loss that often occurs after weight-loss surgery. The study is a collaboration by researchers at Wake Forest University School of Medicine and Wake Forest University’s health and exercise science department.
“Bariatric surgery offers many health benefits to patients with severe obesity, but weight loss can also be associated with a decrease in bone mass, which increases fracture risk,” said Ashley Weaver, Ph.D., associate professor of biomedical engineering at Wake Forest University School of Medicine and the principal investigator leading the HR-pQCT ancillary study to the STRONG BONES trial.
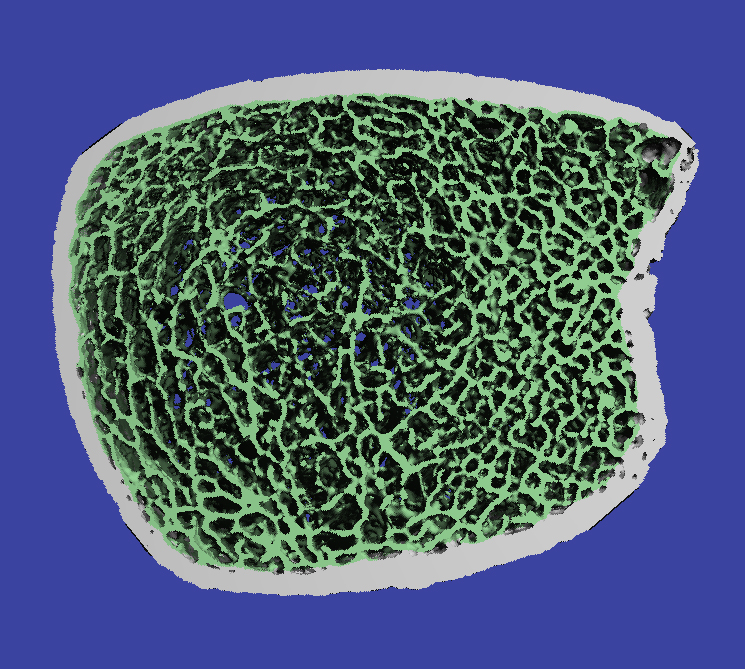
The HR-pQCT scanner is a state-of-the-art device, recently installed at Atrium Health Wake Forest Baptist Medical Center and the first in North Carolina, that uses a low-radiation approach to assess bone mineral density and bone microarchitecture.
“Unlike other available imaging, HR-pQCT allows high-resolution novel image processing techniques that can provide precise images with detailed characterization of bone,” Weaver said. “By analyzing this bone microarchitecture, we have valuable insight into fracture risk and skeletal fragility.”
The STRONG BONES study is recruiting 120 participants over the age of 40 who are undergoing sleeve gastrectomy, a procedure in which part of the stomach is removed to make it smaller and produce robust weight loss. Participants will receive six months of risedronate medication or placebo with all participants receiving HR-pQCT imaging before, during and after treatment.
“We are able to combine this high-resolution imaging with computational modeling to understand how bone strength changes following surgery and in response to the intervention,” said Caitlyn Collins, Ph.D., assistant professor of biomedical engineering and mechanics at Virginia Tech and a co-investigator on the ancillary study to the STRONG BONES trial. “This gives us a more in-depth understanding of how and where risedronate may be affecting bone remodeling in our study participants.”
STRONG BONES is led by Kristen M. Beavers, Ph.D., associate professor of health and exercise science at Wake Forest University and Jamy Ard, M.D., professor of epidemiology and prevention at Wake Forest University School of Medicine and co-director of the Atrium Health Wake Forest Baptist Weight Management Center.
“By partnering with the parent study, we hope to gain insight into the biology of bone loss attributed to bariatric surgery, its associated weight loss and whether risedronate is an effective countermeasure,” Weaver said. “Our findings could change clinical guidelines and reduce long-term fracture risk in this patient population.”
Recruitment is currently ongoing. Those who wish to learn more or participate in the study may contact the STRONG BONES study coordinator by calling 336-758-4078 or visiting the study website.
Media Contact: Myra Wright, mgwright@wakehealth.edu
